High Containment in the chemical and pharmaceutical industry: how to protect operators health and preserve product quality

Continuous research to provide effective treatments for serious illnesses has led scientists to develop drugs with increasingly potent active ingredients, called HPAPIs (High Potency Active Pharmaceutical Ingredients).
These potent drugs, require high containment systems to be managed in order to protect not only the health of the operators employed in the production phase, but also to avoid cross-contamination, and thus, preserve the quality of the product.
In fact, apart from various regulations provided by each European country regarding safety at work, other entities such as the European Medicines Agency (EMA) and the Food and Drug Administration of the States States (FDA), have become increasingly restrictive and rigorous with respect to GMP specifications, which require methods, equipment, and structures that can meet quality and safety requirements.
What is high-containment and when is it needed?
The term “high-containment” refers to a series of measures taken to limit the spread of particular substances (for example HPAPI) and thus, protect the process environment, including operators health.
At the moment there are no unified European standards, but in 2017 a German subsidiary of ISPE association (International Society for Pharmaceutical Engineering) published the “Containment Manual”, which quickly became the benchmark for manufacturers and users of the sector.
The so-called “containment pyramid” is use to represent hazardous levels. Designed to satisfy both current and projected industry standards, it provides pharmaceutical and chemical companies with a clear guide to assessing the level of containment required to safely handle differing hazardous materials.
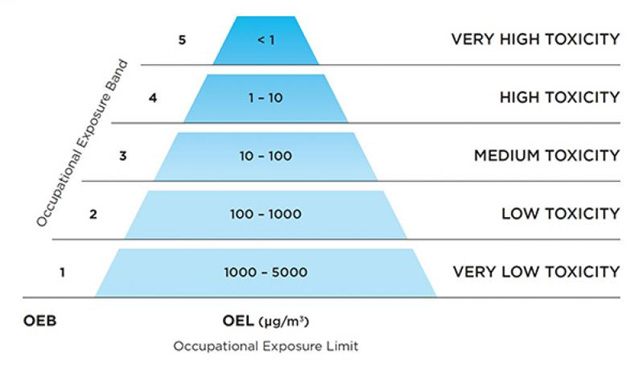
What is OEL?
OEL (Occupational Exposure Limit) is an upper limit on the acceptable air concentration of a hazardous substance in workplace air for a particular substance or group of substances to which workers can be exposed throughout their working lifetime (for 8 hours per day, 5 days a week, for 40 years) without developing adverse health effects.
What is OEB?
OEB (Occupational Exposure Band) is a process intended to accurately assign chemicals into specific categories (bands), each corresponding to a range of exposure concentrations designed to protect worker health. These bands are assigned based on a chemical’s toxicological potency and the adverse health effects associated with exposure to the chemical.
What does Sterivalves do for containment?
As valves manufacturers and technical solutions provider of devices for the pharmaceutical industry, we clearly understand that our customers’ needs are paramount, especially when these concern their health and safety.
As market requested a high containment solution for potent product transfer, our cutting edge valve was born: the STERISPLIT valve.
Made of stainless steel, available from size ND50 to ND200, manual or automatic, Steriplit valve is the ideal product to use in those processes where high containment and / or prevention of cross-contamination are essential requirements.
Need more information? Visit our webpage:
https://sterivalves.eu/en/product/high-containment-sterisplit/



